Evolutionary ‘time travel’ reveals enzyme’s origins, achievable upcoming types
“The difference involving the previous, existing and foreseeable future is only a stubbornly persistent illusion,” Albert Einstein wrote. Possibly this is nowhere more obvious than in protein evolution, where past and present versions of the exact same enzyme exist in diverse species these days, with implications for foreseeable future enzyme structure. Now, researchers have used evolutionary “time travel” to master how an enzyme evolved around time, from 1 of Earth’s most historical organisms to modern-day-day human beings.
The scientists will existing their success right now at the tumble conference of the American Chemical Modern society (ACS).
“If a individual lives in present-day Rome, they might want to study about ancient Rome to improved fully grasp who they are,” claims Magnus Wolf-Watz, Ph.D., the project’s principal investigator. “In the very same way, we can glance backward in time at much more historical kinds of enzymes to realize how the proteins are operating these days and how we could possibly engineer new versions in the long term.”
Wolf-Watz, who is at Umeå College in Sweden, appeared back again some 2 to 3 billion many years to primitive organisms recognized as archaea. These one-celled lifetime sorts, which nevertheless exist these days, have features of both equally prokaryotes (microorganisms, which lack a mobile nucleus) and eukaryotes (organisms like vegetation, animals and fungi that have a nucleus in their cells). A branch of archaea recognised as the Asgard phylum, uncovered in 2015, comprises the closest acknowledged ancestors to eukaryotic cells. 4 styles of Asgard archaea have been determined, including Odin archaea, located in hydrothermal vents deep in the Atlantic Ocean.
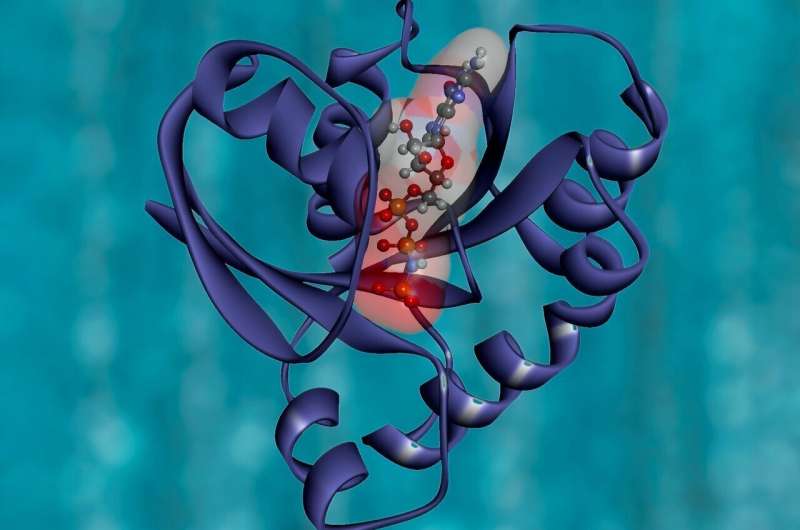
Odin archaea have an enzyme referred to as adenylate kinase (AK), which is also uncovered in prokaryotes and eukaryotes. Wolf-Watz earlier analyzed two human styles of this enzyme, AK1 and AK3. Both equally are critical in protecting the electricity stability in cells, but AK1 is in the cytoplasm, exactly where it transfers a phosphate team from adenosine triphosphate (ATP, the primary power provider in cells) to adenosine monophosphate (AMP). In distinction, AK3 resides within just mitochondria, exactly where it transfers a phosphate group from guanosine triphosphate (GTP, a molecule very similar to ATP but with unique roles) to AMP.
Wolf-Watz’s workforce utilized X-ray crystallization and nuclear magnetic resonance spectroscopy to examine the structures of AK1 and AK3, locating that though the enzymes are incredibly comparable, they have a delicate distinction in a quick loop area that results in AK1 to desire ATP and AK3 to favor GTP. “Now we can consider any AK enzyme, glimpse at the composition of that loop, and forecast whether it can be heading to use ATP or GTP,” Wolf-Watz states. The following stage was to study a extra ancient variation of an AK enzyme –– from Odin archaea –– to master how AK1 and AK3 developed to prefer diverse nucleotide substrates.
The scientists purified the archaea AK and established its structure. They uncovered that the loop critical for discriminating ATP and GTP is a great deal for a longer time in the archaeal enzyme, and it has chemical teams that can bind possibly nucleotide. “What we identified is an early ancestor of the human AKs that incorporates two capacities –– it can use both ATP and GTP,” Wolf-Watz states. “For the duration of the training course of evolution, it grew to become specialised to develop into unique for a person or the other, depending on the cellular compartment wherever it resides.” The archaea AK can essentially use all naturally transpiring nucleotide triphosphates (NTPs). “We’ve uncovered a universal NTP binding motif that could be a making block for the potential structure of novel enzymes,” Wolf-Watz states.
The archaeal AK is made up of 3 copies of the enzyme (regarded as a trimer) that bind to just about every other by means of a helical composition. In human AKs, a mutation in this area tends to make the enzyme copies unable to stick to each individual other. The human enzymes, which perform independently, are almost 1,000-fold far more active. The trimer could have been much more steady in the extraordinary atmosphere of hydrothermal vents, but the human enzymes might have traded this thermostability for bigger exercise, which is essential in a cooler natural environment, Wolf-Watz suggests.
Future, the researchers want to engineer novel enzymes that could be beneficial in natural and organic synthesis or drug advancement. They also want to take a look at other enzymes from Odin archaea and study how they could possibly have evolved in excess of the eons. “We analyzed one enzyme and produced this excellent discovery,” Wolf-Watz states. “Of study course, there’s more to uncover. It truly is like we’re digging through a treasure upper body.”
A lot more details:
Evolutionary origins of enzymatic specificity and dynamics, ACS Slide 2021.
Quotation:
Evolutionary ‘time travel’ reveals enzyme’s origins, achievable upcoming layouts (2021, August 25)
retrieved 25 August 2021
from https://phys.org/news/2021-08-evolutionary-reveals-enzyme-future.html
This document is topic to copyright. Apart from any good dealing for the function of private study or analysis, no
part may be reproduced without the need of the prepared authorization. The content material is offered for details purposes only.







